Zanubrutinib is an oral small-molecule inhibitor of Bruton’s tyrosine kinase (BTK). It is used to treat mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients who have received at least one prior therapy.
Zanubrutinib was invented by BeiGene and was approval by the FDA in November 2019 based on clinical trial results that demonstrated an 84% overall response rate from Zanubrutinib therapy in patients with MCL.[1] It is currently marketed under the trade name BRUKINSA™ and is available as oral capsules.

What is BTK?
Bruton tyrosine kinase (BTK), a non-receptor tyrosine kinase encoded by the BTK gene, was officially discovered and named in the 1990s, and is expressed only in B cells and myeloid cells. BTK is a key component of THE B lymphocyte receptor (BCR) signaling pathway, and plays a key role in the BCR signaling pathway, and has an important influence on the proliferation, differentiation, and apoptosis of B cells. In malignant B-cell lymphoma, the BCR signaling pathway is overactive, which inhibits the normal differentiation and apoptosis of B cells and promotes abnormal proliferation, leading to the accumulation of malignant B lymphocytes in bone marrow, secondary lymphoid organs, and blood. Therefore, BTK also plays an important role in the proliferation and survival of malignant B cells.
What does BTK look like?
There are 659 residues of human BTK, which are divided into five domains: PH, TH, SH3, SH2, and the catalytic domain. The PH domain mainly mediates the protein-phospholipid (PIP3) and protein-protein interaction, which is related to the localization of BTK. TH is a proline-rich Tec homology domain that contains a zinc finger motif and plays an important role in protein activity and stability. The SH3 and SH2 domains have binding functions and contain an autophosphorylation site Y233. The catalytic domain contains the phosphorylation site Y551, the ATP binding pocket, and the important covalent inhibitor binding site C481.
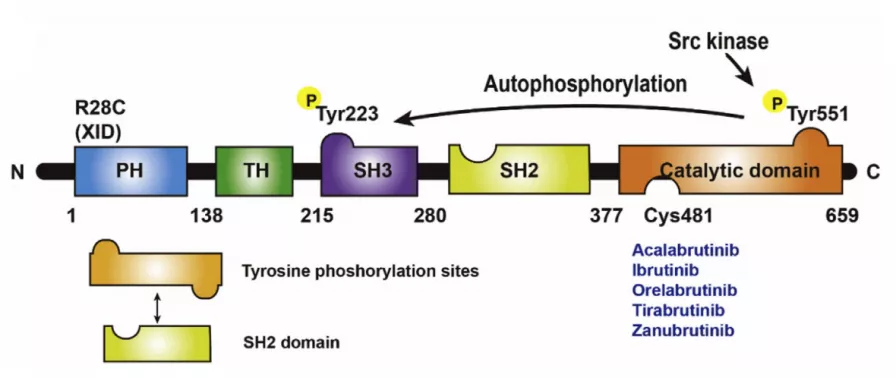
How many types of BTK inhibitors?
- Irreversible covalent inhibitors: Ibrutinib, Acalabrutinib, Zanubrutinib, Tirabrutiinib, Orelabrutinib, TG-1701, TG-1702, DTRMWXHS-12, Spebrutinib,[3] ect.
- Reversible non-covalent inhibitors: Vecabrutinib, Pirtobrutinib, Fenebrutibib, ARQ-531, Xmu-MP-3, CB1763, GGNE-431, CGI-1746,[4] ect.
- BTK-PROTAC: MT-802, SJF620, P13I, L18I, CJH-005-067, DD-04-015,[5] ect.
How does Zanubrutinib work?
Zanubrutinib inhibits BTK by forming a covalent bond with Cys481 residue in the adenosine triphosphate (ATP)–binding pocket of BTK, which is the enzyme’s active site. This binding specificity is commonly seen with other BTK inhibitors. Due to this binding profile, Zanubrutinib may also bind with varying affinities to related and unrelated ATP-binding kinases that possess a cysteine residue at this position.[7] By blocking the BCR signaling pathway, Zanubrutinib inhibits the proliferation, trafficking, chemotaxis, and adhesion of malignant B cells, ultimately leading to reduced tumor size.[6] Zanubrutinib was also shown to downregulate programmed death-ligand 1 (PD-1) expression and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) on CD4+ T cells.[8]
References:
[1]FDA grants accelerated approval to zanubrutinib for mantle cell lymphoma.https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-zanubrutinib-mantle-cell-lymphoma
[2]Liu J, Chen C, Wang D, et al. Emerging small-molecule inhibitors of the Bruton’s tyrosine kinase (BTK): Current development. Eur J Med Chem. 2021;217:113329. doi:
[3]Burger JA, Wiestner A. Targeting Bcell receptor signalling in cancer: preclinical and clinical advances. Nat Rev Cancer. 2018;18(3):148-167. doi:10.1038/nrc.2017.121.
[4]Gu, D., Tang, H., Wu, J. et al. Targeting Bruton tyrosine kinase using non-covalent inhibitors in B cell malignancies. J Hematol Oncol 14, 40 (2021). https://doi.org/10.1186/s13045-021-01049-7
[5]George B, Chowdhury SM, Hart A, et al. Ibrutinib Resistance Mechanismsand Treatment Strategies for B-Cell lymphomas. Cancers(Basel). 2020;12(5):1328. doi:
[6]FDA Approved Drug Products: Brukinsa (zanubrutinib) capsules. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213217s000lbl.pdf
[7]Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, Harrup R, Johnston PB, Marlton P, Munoz J, Seymour JF, Simpson D, Tedeschi A, Elstrom R, Yu Y, Tang Z, Han L, Huang J, Novotny W, Wang L, Roberts AW: Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019 Sep 12;134(11):851-859. doi: 10.1182/blood.2019001160. Epub 2019 Jul 24
[8]Zou YX, Zhu HY, Li XT, Xia Y, Miao KR, Zhao SS, Wu YJ, Wang L, Xu W, Li JY: The impacts of zanubrutinib on immune cells in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Hematol Oncol. 2019 Oct;37(4):392-400. doi: 10.1002/hon.2667. Epub 2019 Sep 13.

